MEDIUM
Earn 100
From the following molecules, how many of them have atoms in the same plane.
(a)
(b)
(c)
(d)
50% studentsanswered this correctly
Important Questions on Chemical Bonding and Molecular Structure
HARD
MEDIUM
MEDIUM
EASY
MEDIUM
EASY
EASY
EASY
MEDIUM
EASY
EASY
How many (i) hybridised carbon atoms and (ii) bonds are present in the following compound?
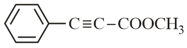
EASY
MEDIUM
MEDIUM
EASY
MEDIUM
EASY
EASY
EASY
(i)
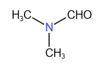 (ii)
(ii) 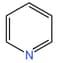
(iii) (iv)
MEDIUM

