HARD
Earn 100
From the following reaction choose reaction that are intramolecular redox reactions but not disproportionation reactions
(a)
(b)
(c)
(d)
50% studentsanswered this correctly
Important Questions on Redox Reactions
EASY
HARD
The redox reaction among the following is:
MEDIUM
EASY
EASY
Which of the following statements is correct ?
EASY
HARD
EASY
MEDIUM
(Unbalanced)
EASY
EASY
EASY
EASY
Which is reduced in the following reaction
MEDIUM
Strongest reducing agent among the following is
(i)
(ii)
(iii)
(iv)
EASY
EASY
In the following resonance structures, the curved arrow indicates that electrons are shifted from
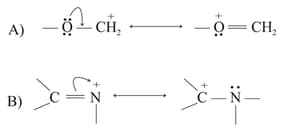
EASY
Among the following, the strongest reducing agent is:
EASY
Role of hydrogen peroxide in the above reactions is respectively:
EASY
EASY

