Fructose is an isomer of glucose, but they differ with regard to one functional group and hence in their redox properties.Identify the functional group present in fructose, but not glucose.

Important Questions on Biochemistry
Lactulose is a synthetic, non-digestible disaccharide that is used in the treatment of chronic constipation and liver disease. This disaccharide contains the residues of galactose and fructose. The formula of α-lactulose is given below
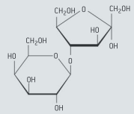
Identify the glycosidic link in lactulose by drawing a circle around it.
Lactulose is a synthetic, non-digestible disaccharide that is used in the treatment of chronic constipation and liver disease. This disaccharide contains the residues of galactose and fructose. The formula of α-lactulose is given below
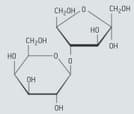
Suggest whether lactulose is a reducing or non-reducing sugar. Explain your answer.
In making candy or sugar syrup, sucrose is boiled in water with a small amount of organic acid, such as citric acid from lemon juice. Explain why the product mixture tastes sweeter than the initial sucrose solution.
