Galvanic cells converts electrical energy into chemical energy.
Important Questions on Electrochemistry
Represent a cell consisting of | half cell and | half cell and write the cell reaction.
At , the of the galvanic cell mentioned below is
For the cell , when the concentration of is times the concentration of , the expression for is
Faraday's constant, universal gas constant, temperature,
Depict the galvanic cell in which the reaction takes place. Further show, which of the electrode is negatively charged.
.
The reaction occurs in which of the given galvanic cell?
Some materials are given below:
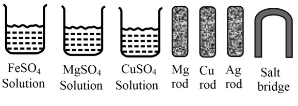
From the given materials, choose the appropriate ones to construct a galvanic cell and draw the diagram of the cell.
(Order of reactivity: )
The following reaction takes place at in an electrochemical cell involving two metals and ,
with and in the respective half-cells, the cell EMF is . The equilibrium constant of the reaction is closest to;
,
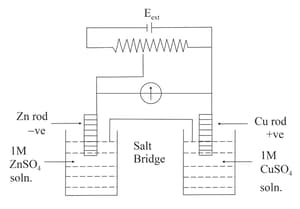
Identify the incorrect statement from the options below for the above cell:

