Give reason why beryllium does not exhibit coordination numbers more than four.
Important Questions on The s-Block Elements
Reason (R) : Both and have almost same ionic radius
The correct option among the following is
Given below are two statements:
Statement I : The chlorides of and have Cl-bridged structure. Both are soluble in organic solvents and act as Lewis bases.
Statement II: Hydroxides of and dissolve in excess alkali to give beryllate and aluminate ions. In the light of the above statements. Choose the correct answer from the options given below.
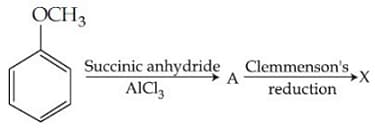
X is :
Match list-I with list-II :
| (a) | (i) | Treatment of cancer | |
| (b) | (ii) | Extraction of metals | |
| (c) | (iii) | Incendiary bombs and signals | |
| (d) | (iv) | Windows of X-ray tubes | |
| (v) | Bearings for motor engines. |
Choose the most appropriate answer, the option given below :
Match List with List
| List | List | ||
| (a) | (i) | Antacid | |
| (b) | (ii) | Cement | |
| (c) | (iii) | Bleach | |
| (d) | (iv) | Plaster of paris |
Choose the most appropriate answer from the
Among the statement (I – IV), the correct ones are:
(I) Be has smaller atomic radius compared to Mg.
(II) Be has higher ionization enthalpy than AI.
(III) Charge/radius ratio of Be is greater than that of Al.
(IV) Both Be and Al form mainly covalent compounds

