MEDIUM
10th CBSE
IMPORTANT
Earn 100
Give reasons for the following:
(i) Oxidation of ethanol with produces ethanal while ethanol when oxidised with alkaline produces ethanoic acid.
(ii) Alcohol supplied for industrial purposes is mixed with copper sulphate.

Important Questions on Carbon and its Compounds
MEDIUM
10th CBSE
IMPORTANT
(i)
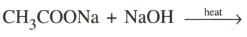
(ii)
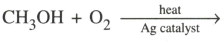
MEDIUM
10th CBSE
IMPORTANT
MEDIUM
10th CBSE
IMPORTANT
MEDIUM
10th CBSE
IMPORTANT
ii)An organic compound A is a constituent of antifreeze. The compound on heating with oxygen forms another compound “B” that has a molecular formula . Identify the compound “A” and “B”. Write the chemical equation of the reaction that takes place to form the compound “B”.
HARD
10th CBSE
IMPORTANT
MEDIUM
10th CBSE
IMPORTANT
It forms monochloro addition product “Y” with gaseous HCI. Molecular weight of “Y” is 64.5. Identify the compounds “X” and “Y”. Write the chemical reaction of “X” with bromine water and HCl gas.
MEDIUM
10th CBSE
IMPORTANT
MEDIUM
10th CBSE
IMPORTANT
For the reaction (i) Name the main product (ii) Write the chemical equation.
