Give reasons for the following: The ratio of hydrogen and oxygen formed at the cathode and anode is by volume.

Important Questions on Electrolysis
Give reasons for the following: In the electrolysis of acidified water, dilute sulphuric acid is preferred to dilute nitric acid for acidification.
Give reasons for the following: Ammonia is unionised in the gaseous state but in the aqueous solution, it is a weak electrolyte.
Give reason for the following: A graphite anode is preferred to other inert electrodes during electrolysis of fused lead bromide.
Give reason for the following: For electroplating with silver, silver nitrate is not used as electrolyte.
Give reasons for the following: Carbon tetrachloride is a liquid but does not conduct electricity.
Give reasons for the following: Potassium is not extracted by electrolysis of its aqueous salt solution.
Copy and complete the following table which refers to two practical applications of electrolysis
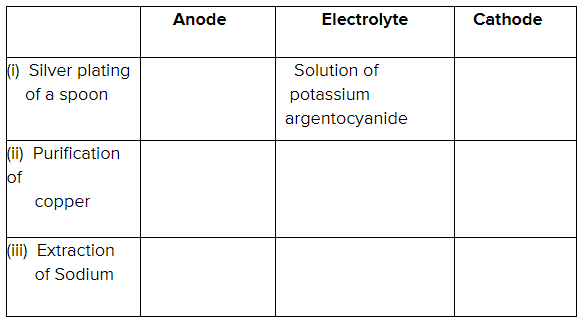
Write equations of reactions taking place at anode for silver plating.
