EASY
12th ICSE
IMPORTANT
Earn 100
Give the half-cell and net reactions in the Leclanche cell.

Important Questions on Electrochemistry
MEDIUM
12th ICSE
IMPORTANT
EASY
12th ICSE
IMPORTANT
HARD
12th ICSE
IMPORTANT
The standard reduction potential in acidic condition is and , respectively, for and couples.
MEDIUM
12th ICSE
IMPORTANT
From the following molar conductivities at infinite dilution: for ; ; . Calculate for .
MEDIUM
12th ICSE
IMPORTANT
MEDIUM
12th ICSE
IMPORTANT
EASY
12th ICSE
IMPORTANT
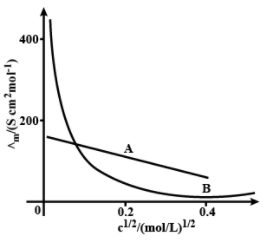
The following curve is obtained when molar conductivity, is plotted against the square root of concentration, along and axis respectively for the two electrolytes and . What can you say about the nature of these two electrolytes?
MEDIUM
12th ICSE
IMPORTANT
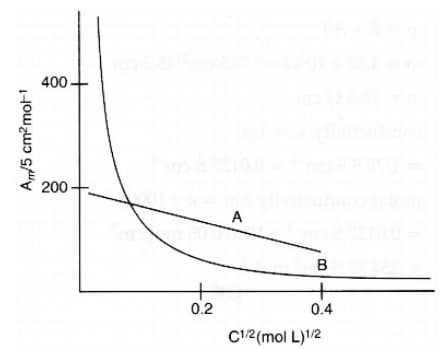
The following curve is obtained when molar conductivity, is plotted against the square root of concentration, along and -axis respectively for the two electrolytes and . Account for the increase in for the electrolytes and with dilution.