EASY
Earn 100
Give the reaction of magnesium with boiling water or steam.
Important Questions on Periodicity
MEDIUM
The acidic, basic and amphoteric oxides, respectively, are
MEDIUM
In the following sets of reactants which two sets best exhibit the amphoteric character of Al2O3 . xH2O ?
EASY
The metal that gives hydrogen gas upon treatment with both the acid as well as the base is
EASY
Which among the following oxides is the most basic?
MEDIUM
Write a chemical equation for the reaction taking place in the test tube. How does the colour of the solution change? What is the change in colour of iron nails?
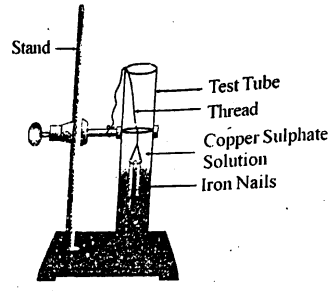
EASY
Match the following :
| Oxide | Nature |
| (a) | (i) Basic |
| (b) | (ii) Neutral |
| (c) | (iii) Acidic |
| (d) | (iv) Amphoteric |
Which of the following is correct option?
HARD
The option(s) with only amphoteric oxides is(are):
EASY
Identify the element that forms amphoteric oxide:
EASY
What is the gas liberated when the metals react with dilute acids?
EASY
Which of the following metal in combination with oxygen would yield basic metal oxide?
EASY
Identify the correct order of electronegativity for and .
MEDIUM
An element reacts with oxygen to give a compound with a high melting point. This compound is also soluble in water. The element is likely to be-
EASY
Identify the correct statement from the following.
MEDIUM
Three elements and are in the period of the periodic table. The oxides of and , respectively, are basic, amphoteric and acidic. The correct order of the atomic numbers of and is:
EASY
is attacked by which one/ones of the following?
MEDIUM
The gas evolved on heating and with concentrated , on hydrolysis gives a white gelatinous precipitate. The precipitate is:
EASY
. What type of chemical reaction is shown in the above equation?
HARD
Differentiate between metals and non-metals on the basis of their chemical properties.
MEDIUM
Choose the acidic oxides, basic oxides and neutral oxides from the following:
EASY
What is your observation in the following case:
Burning of magnesium in air.

