HARD
JEE Main
IMPORTANT
Earn 100
Given below are the half - cell reactions :
The for will be :
(a) ; the reaction will not occur
(b) ; the reaction will occur
(c) ; the reaction will not occur
(d) ; the reaction will occur
42.11% studentsanswered this correctly

Important Questions on Electrochemistry
MEDIUM
JEE Main
IMPORTANT
For the given cell;
change in Gibbs energy is negative, it :
MEDIUM
JEE Main
IMPORTANT
Potassium chlorate is prepared by the electrolysis of in basic solution . If only of the current is utilized in the reaction, the time (rounded to the nearest hour) required to produce of using a current of is (Given :; molar mass of )
EASY
JEE Main
IMPORTANT
The variation of molar conductively with concentration of an electrolyte (X) in aqueous solution is shown in the given figure.
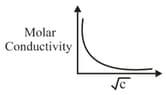
The electrolyte X is :
EASY
JEE Main
IMPORTANT
An oxidation-reduction reaction in which 3 electrons are transferred has a of at The value of (in V) is .
EASY
JEE Main
IMPORTANT
Two Faraday of electricity is passed through a solution of . The mass of copper deposited at the cathode is: (Atomic mass of Cu = 63.5 amu)
MEDIUM
JEE Main
IMPORTANT
of a waste solution obtained from the workshop of a goldsmith contains and . The solution was electrolyzed at by passing a current of for minutes. The metal/metals electrodeposited will be :
EASY
JEE Main
IMPORTANT
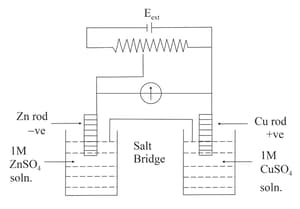
Identify the incorrect statement from the options below for the above cell:
EASY
JEE Main
IMPORTANT
Galvanization is applying a coating of:
