Given below are two statement : one is labelled as Assertion and the other is labelled as Reason .
Assertion is adsorbed to a large extent than on activated charcoal.
Reason : has a higher critical temperature than
In the light of the above statements, choose the most appropriate answer from the options given below.
Assertion is adsorbed to a large extent than on activated charcoal.
correct explanation fo
explanation of .

Important Questions on States of Matter: Gases and Liquids
The above reaction is carried out in a vessel starting with partial pressure , and . When the reaction is complete, the total pressure in the reaction vessel is _____ .
(Round off of the nearest integer).
Two flasks I and II shown below are connected by a valve of negligible volume.
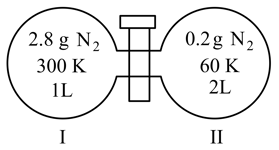
When the valve is opened, the final pressure of the system in bar is . The value of is (Integer answer)
[Assume : Ideal gas; bar : Molar mass of
The unit of the van der Waals gas equation parameter in is:
A home owner uses of methane gas, (assume is an ideal gas) in a year to heat his home. Under the pressure of atm and , mass of gas used is . The value of is ______. (Nearest integer)
(Given )
