MEDIUM
JEE Main
IMPORTANT
Earn 100
Given below are two statements:
Statement I : The identification of is carried out by dimethyl glyoxime in the presence of .
Statement II :The dimethyl glyoxime is a bidentate neutral ligand.
In the light of the above statements, choose the correct answer from the options given below:
Statement I : The identification of is carried out by dimethyl glyoxime in the presence of .
(a)Statement is false but Statement is true.
(b)Statement is true but Statement is false.
(c)Both Statement and Statement are true.
(d)Both Statement and Statement are false.
17.14% studentsanswered this correctly

Important Questions on Coordination Compounds
MEDIUM
JEE Main
IMPORTANT
The hybridization and magnetic nature of and respectively are :
EASY
JEE Main
IMPORTANT
The calculated magnetic moments (spin only value) for species and respectively are:
MEDIUM
JEE Main
IMPORTANT
Spin only magnetic moment of an octahedral complex of in the presence of a strong field ligand in BM is:
MEDIUM
JEE Main
IMPORTANT
Which one of the following species doesn't have a magnetic moment of (spin only value)?
EASY
JEE Main
IMPORTANT
An aqueous solution of was heated with excess sodium cyanide in presence of strong oxidizing agent to form The total change in number of unpaired electrons on metal centre is ___________.
MEDIUM
JEE Main
IMPORTANT
The correct order of intensity of colors of the compounds is:
HARD
JEE Main
IMPORTANT
Simplified absorption spectra of three complexes ((i) and (ii) and (iii)) of ion are provided below; their values are marked as and respectively. The correct match between the complexes and their values is:
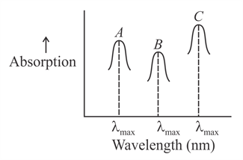
(i)
(ii)
(iii)
EASY
JEE Main
IMPORTANT
The one that is not expected to show isomerism is:
