EASY
JEE Main
IMPORTANT
Earn 100
Given below are two statements: one is labelled as Assertion and the other is labelled as Reason .
Assertion : Dipole-dipole interactions are the only non-covalent interactions, resulting in hydrogen bond formation.
Reason : Fluorine is the most electronegative element and hydrogen bonds in are symmetrical.
In the light of the above statements, choose the most appropriate answer from the options given below:
(a) is false but is true
(b)Both and are true but is NOT the correct explanation of
(c) is true but is false
(d)Both and are true and is the correct explanation of
50% studentsanswered this correctly

Important Questions on Chemical Bonding and Molecular Structure
MEDIUM
JEE Main
IMPORTANT
The bond order and magnetic behaviour of ion are, respectively :
HARD
JEE Main
IMPORTANT
In the following the correct bond order sequence is:
MEDIUM
JEE Main
IMPORTANT
Identify the species having one -bond and maximum number of canonical forms from the following :
EASY
JEE Main
IMPORTANT
The number of sigma bonds in
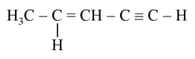 is _______.
is _______.MEDIUM
JEE Main
IMPORTANT
The correct order of bond dissociation enthalpy of halogens is:
MEDIUM
JEE Main
IMPORTANT
Which among the following species has unequal bond lengths?
MEDIUM
JEE Main
IMPORTANT
According to molecular orbital theory, the species among the following that does not exist is:
EASY
JEE Main
IMPORTANT
The correct set from the following in which both pairs are in correct order of melting point is:
