MEDIUM
JEE Main
IMPORTANT
Earn 100
Given below are two statements: one is labelled as Assertion and the other is labelled as Reason.
Assertion: A mixture contains benzoic acid and napthalene. The pure benzoic acid can be separated out by the use of benzene.
Reason: Benzoic acid is soluble in hot water.
In the light of the above statements, choose the most appropriate answer from the options given below.
(a)Both Assertion and Reason are true and Reason is the correct explanation of Assertion.
(b)Both Assertion and Reason are true but Reason is not the correct explanation of Assertion.
(c)Assertion is true but Reason is false.
(d)Assertion is false but Reason is true.
50% studentsanswered this correctly

Important Questions on Aldehydes, Ketones and Carboxylic Acids
HARD
JEE Main
IMPORTANT
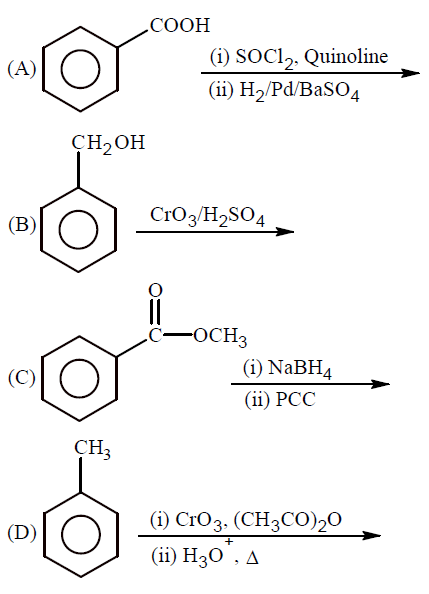
Which of the following conditions or reaction sequence will NOT give acetophenone as the major product?
HARD
JEE Main
IMPORTANT
Which of the following ketone will NOT give enamine on treatment with secondary amines? [where is ]
MEDIUM
JEE Main
IMPORTANT
Toluene can be easily converted into benzaldehyde by which of the following reagents?
MEDIUM
JEE Main
IMPORTANT
The reagent, from the following, which converts benzoic acid to benzaldehyde in one step is
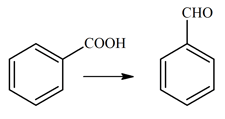
MEDIUM
JEE Main
IMPORTANT
The final product '' in the following reaction sequence
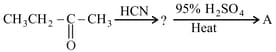
MEDIUM
JEE Main
IMPORTANT
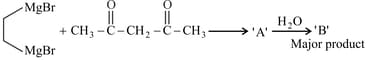
Consider the above reaction sequence and identify the product .
MEDIUM
JEE Main
IMPORTANT
'' and '' respectively are
A. Ethane--dicarbaldehyde Glyoxal/Oxaldehyde
B -oxohexanal
HARD
JEE Main
IMPORTANT
Which of the following reactions will yield benzaldehyde as a product?