Given that the reduced temperature, the reduced pressure, the reduced volume,
Thus, it can be said that the reduced equation of state may be given as-
Important Questions on States of Matter

(Latent heat of ice is and )

The correct option(s) is (are)
Given below are two statement : one is labelled as Assertion and the other is labelled as Reason .
Assertion is adsorbed to a large extent than on activated charcoal.
Reason : has a higher critical temperature than
In the light of the above statements, choose the most appropriate answer from the options given below.
The combination of plots which does not represent isothermal expansion of an ideal gas is




The number of statement's, which are correct with respect to the compression of carbon dioxide from point (a) in the Andrews isotherm from the following is _________.
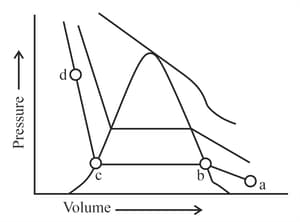
A. Carbon dioxide remains as a gas upto point (b)
B. Liquid carbon dioxide appears at point (c)
C. Liquid and gaseous carbon dioxide coexist between points (b) and (c)
D. As the volume decreases from (b) to (c), the amount of liquid decreases
Among the following gases, the order of liquefiability is
a)
b)
c)
d)

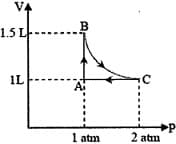
The above diagram represents the thermodynamic cycle of an engine, operating with an ideal mono-atomic gas. The amount of heat, extracted from the source in a single cycle, is:
(R = 8.314 J/mol K) (ln7.5 = 2.01)
[Heat of fusion of ice ; Specific heat of water ]
Which of the following gases can be absorbed in more proportion?
| Gas | ||||
|---|---|---|---|---|
Which gas is expected to have the highest critical temperature?
