Given two statements below :
Statement I : In molecule the covalent radius is double of the atomic radius of chlorine.
Statement II : Radius of anionic species is always greater than their parent atomic radius.
Choose the most appropriate answer from options given below

Important Questions on Classification of Elements and Periodicity in Properties
Outermost electronic configurations of four elements are given below:
(A)
(B)
(C)
(D)
The correct order of first ionisation enthalpy for them is
In which of the following pairs, electron gain enthalpies of constituent elements are nearly the same or identical?
(A) and
(B) and
(C) and
(D) and
Choose the correct answer from the options given below:
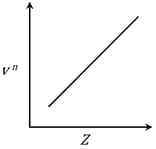
It is observed that characteristic X-ray spectra of elements show regularity. When frequency to the power 'n' i.e. of X-rays emitted is plotted against atomic number , the following graph is obtained.
The value of 'n' is