MEDIUM
Earn 100
Gold Nucleus can decay into mercury nucleus by two decay schemes shown in figure. (i) It can emit a particle and come to ground state by either emitting one ray or emitting two rays (ii) It can emit one particle and come to ground state by emitting ray. Atomic masses: The energy levels of the nucleus are shown in figure.
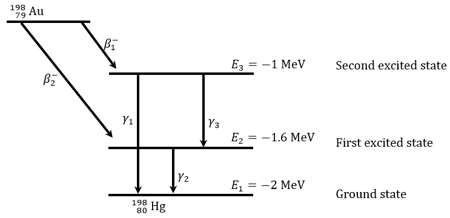

(a)The maximum kinetic energy of emitted particles
(b)The maximum kinetic energy of emitted particles
(c)The maximum kinetic energy of emitted particles
(d)The maximum kinetic energy of emitted particles
50% studentsanswered this correctly
Important Questions on Atoms and Nuclei
EASY
HARD
EASY
(velocity of light, is )
MEDIUM
MEDIUM
Arrange rays in ascending order of their penetrating power.
HARD
[Given: atomic mass of atomic mass of atomic mass of particle is speed of the light]
MEDIUM
EASY
What is the binding energy of whose atomic mass is
Mass of proton
Mass of neutron
(Neglect the electron mass) (Assume )
MEDIUM
Compare the charge and ionising power of rays. Mention one use of radioactivity.
EASY
EASY
EASY
MEDIUM
HARD
EASY
EASY
MEDIUM
HARD
EASY
EASY

