Gold has a density of . A mass of of gold contains atoms. Use this information to estimate the volume of a gold atom, and hence its radius. State any assumptions you make.

Important Questions on Atomic Structure and Particle Physics
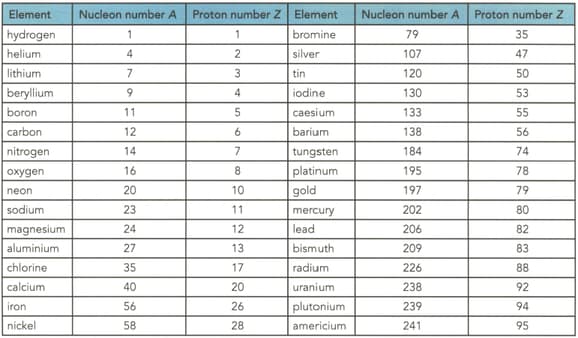
Table shows the proton and nucleon numbers of several nuclei. Determine the number of neutrons in the nuclei of the following elements shown in the table
(a) Nitrogen
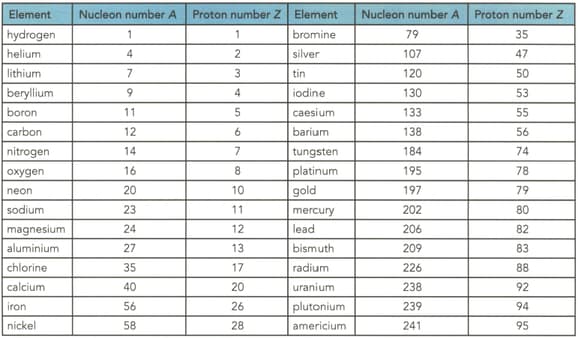
Table shows the proton and nucleon numbers of several nuclei. Determine the number of neutrons in the nuclei of the following elements shown in the table
(b) Bromine
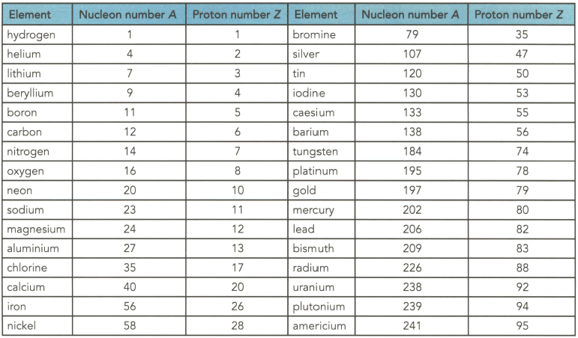
Table shows the proton and nucleon numbers of several nuclei. Determine the number of neutrons in the nuclei of the following elements shown in the table
(c) Silver
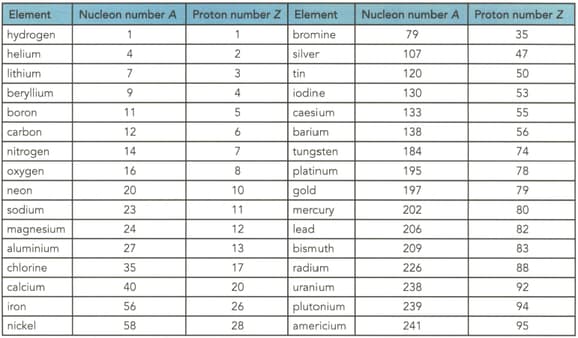
Table shows the proton and nucleon numbers of several nuclei. Determine the number of neutrons in the nuclei of the following elements shown in the table
(d) Gold
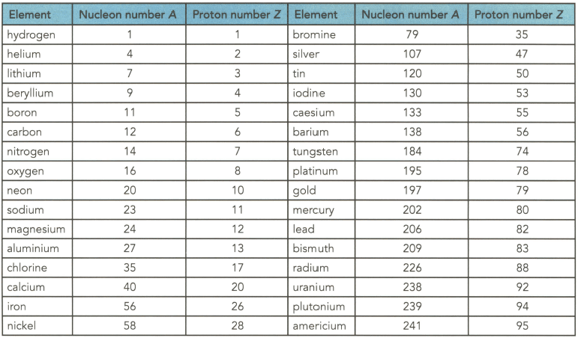
Table shows the proton and nucleon numbers of several nuclei. Determine the number of neutrons in the nuclei of the following elements shown in the table
(e) Mercury
State the charge of each of the following in terms of the elementary charge
(a) Proton
State the charge of each of the following in terms of the elementary charge :
(b) Neutrom
State the charge of each of the following in terms of the elementary charge
(c) Nucleus
