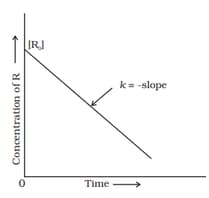
MEDIUM
Earn 100
Graph of half life of zero-order reaction and initial concentration of reactant is a straight line.
50% studentsanswered this correctly
Important Questions on Apply scientific principles and evidence to provide an explanation about the effects of changing the temperature or concentration of the reacting particles on the rate at which a reaction occurs.
MEDIUM
Physical Sciences>Matter and Its Interactions>Apply scientific principles and evidence to provide an explanation about the effects of changing the temperature or concentration of the reacting particles on the rate at which a reaction occurs.>Chemical Reactions - Chemical processes, their rates, and whether or not energy is stored or released can be understood in terms of the collisions of molecules and the rearrangements of atoms into new molecules, with consequent changes in the sum of all bond energies in the set of molecules that are matched by changes in kinetic energy.
MEDIUM
Physical Sciences>Matter and Its Interactions>Apply scientific principles and evidence to provide an explanation about the effects of changing the temperature or concentration of the reacting particles on the rate at which a reaction occurs.>Chemical Reactions - Chemical processes, their rates, and whether or not energy is stored or released can be understood in terms of the collisions of molecules and the rearrangements of atoms into new molecules, with consequent changes in the sum of all bond energies in the set of molecules that are matched by changes in kinetic energy.
MEDIUM
Physical Sciences>Matter and Its Interactions>Apply scientific principles and evidence to provide an explanation about the effects of changing the temperature or concentration of the reacting particles on the rate at which a reaction occurs.>Chemical Reactions - Chemical processes, their rates, and whether or not energy is stored or released can be understood in terms of the collisions of molecules and the rearrangements of atoms into new molecules, with consequent changes in the sum of all bond energies in the set of molecules that are matched by changes in kinetic energy.
EASY
Physical Sciences>Matter and Its Interactions>Apply scientific principles and evidence to provide an explanation about the effects of changing the temperature or concentration of the reacting particles on the rate at which a reaction occurs.>Chemical Reactions - Chemical processes, their rates, and whether or not energy is stored or released can be understood in terms of the collisions of molecules and the rearrangements of atoms into new molecules, with consequent changes in the sum of all bond energies in the set of molecules that are matched by changes in kinetic energy.
MEDIUM
Physical Sciences>Matter and Its Interactions>Apply scientific principles and evidence to provide an explanation about the effects of changing the temperature or concentration of the reacting particles on the rate at which a reaction occurs.>Chemical Reactions - Chemical processes, their rates, and whether or not energy is stored or released can be understood in terms of the collisions of molecules and the rearrangements of atoms into new molecules, with consequent changes in the sum of all bond energies in the set of molecules that are matched by changes in kinetic energy.
MEDIUM
Physical Sciences>Matter and Its Interactions>Apply scientific principles and evidence to provide an explanation about the effects of changing the temperature or concentration of the reacting particles on the rate at which a reaction occurs.>Chemical Reactions - Chemical processes, their rates, and whether or not energy is stored or released can be understood in terms of the collisions of molecules and the rearrangements of atoms into new molecules, with consequent changes in the sum of all bond energies in the set of molecules that are matched by changes in kinetic energy.
EASY
Physical Sciences>Matter and Its Interactions>Apply scientific principles and evidence to provide an explanation about the effects of changing the temperature or concentration of the reacting particles on the rate at which a reaction occurs.>Chemical Reactions - Chemical processes, their rates, and whether or not energy is stored or released can be understood in terms of the collisions of molecules and the rearrangements of atoms into new molecules, with consequent changes in the sum of all bond energies in the set of molecules that are matched by changes in kinetic energy.
HARD
Physical Sciences>Matter and Its Interactions>Apply scientific principles and evidence to provide an explanation about the effects of changing the temperature or concentration of the reacting particles on the rate at which a reaction occurs.>Chemical Reactions - Chemical processes, their rates, and whether or not energy is stored or released can be understood in terms of the collisions of molecules and the rearrangements of atoms into new molecules, with consequent changes in the sum of all bond energies in the set of molecules that are matched by changes in kinetic energy.
EASY
Physical Sciences>Matter and Its Interactions>Apply scientific principles and evidence to provide an explanation about the effects of changing the temperature or concentration of the reacting particles on the rate at which a reaction occurs.>Chemical Reactions - Chemical processes, their rates, and whether or not energy is stored or released can be understood in terms of the collisions of molecules and the rearrangements of atoms into new molecules, with consequent changes in the sum of all bond energies in the set of molecules that are matched by changes in kinetic energy.
EASY
Physical Sciences>Matter and Its Interactions>Apply scientific principles and evidence to provide an explanation about the effects of changing the temperature or concentration of the reacting particles on the rate at which a reaction occurs.>Chemical Reactions - Chemical processes, their rates, and whether or not energy is stored or released can be understood in terms of the collisions of molecules and the rearrangements of atoms into new molecules, with consequent changes in the sum of all bond energies in the set of molecules that are matched by changes in kinetic energy.
HARD
Physical Sciences>Matter and Its Interactions>Apply scientific principles and evidence to provide an explanation about the effects of changing the temperature or concentration of the reacting particles on the rate at which a reaction occurs.>Chemical Reactions - Chemical processes, their rates, and whether or not energy is stored or released can be understood in terms of the collisions of molecules and the rearrangements of atoms into new molecules, with consequent changes in the sum of all bond energies in the set of molecules that are matched by changes in kinetic energy.
Which of the following relation is correct for zero-order reaction?
EASY
Physical Sciences>Matter and Its Interactions>Apply scientific principles and evidence to provide an explanation about the effects of changing the temperature or concentration of the reacting particles on the rate at which a reaction occurs.>Chemical Reactions - Chemical processes, their rates, and whether or not energy is stored or released can be understood in terms of the collisions of molecules and the rearrangements of atoms into new molecules, with consequent changes in the sum of all bond energies in the set of molecules that are matched by changes in kinetic energy.
HARD
Physical Sciences>Matter and Its Interactions>Apply scientific principles and evidence to provide an explanation about the effects of changing the temperature or concentration of the reacting particles on the rate at which a reaction occurs.>Chemical Reactions - Chemical processes, their rates, and whether or not energy is stored or released can be understood in terms of the collisions of molecules and the rearrangements of atoms into new molecules, with consequent changes in the sum of all bond energies in the set of molecules that are matched by changes in kinetic energy.
Examine the graph given below. Identify the integrated rate equation and the order of the reaction corresponding to it.
HARD
Physical Sciences>Matter and Its Interactions>Apply scientific principles and evidence to provide an explanation about the effects of changing the temperature or concentration of the reacting particles on the rate at which a reaction occurs.>Chemical Reactions - Chemical processes, their rates, and whether or not energy is stored or released can be understood in terms of the collisions of molecules and the rearrangements of atoms into new molecules, with consequent changes in the sum of all bond energies in the set of molecules that are matched by changes in kinetic energy.
MEDIUM
Physical Sciences>Matter and Its Interactions>Apply scientific principles and evidence to provide an explanation about the effects of changing the temperature or concentration of the reacting particles on the rate at which a reaction occurs.>Chemical Reactions - Chemical processes, their rates, and whether or not energy is stored or released can be understood in terms of the collisions of molecules and the rearrangements of atoms into new molecules, with consequent changes in the sum of all bond energies in the set of molecules that are matched by changes in kinetic energy.
EASY
Physical Sciences>Matter and Its Interactions>Apply scientific principles and evidence to provide an explanation about the effects of changing the temperature or concentration of the reacting particles on the rate at which a reaction occurs.>Chemical Reactions - Chemical processes, their rates, and whether or not energy is stored or released can be understood in terms of the collisions of molecules and the rearrangements of atoms into new molecules, with consequent changes in the sum of all bond energies in the set of molecules that are matched by changes in kinetic energy.
The given graph is a representation of kinetics of a reaction.
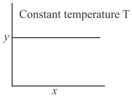
The and axes for zero and first order reactions, respectively are
HARD
Physical Sciences>Matter and Its Interactions>Apply scientific principles and evidence to provide an explanation about the effects of changing the temperature or concentration of the reacting particles on the rate at which a reaction occurs.>Chemical Reactions - Chemical processes, their rates, and whether or not energy is stored or released can be understood in terms of the collisions of molecules and the rearrangements of atoms into new molecules, with consequent changes in the sum of all bond energies in the set of molecules that are matched by changes in kinetic energy.
Derive the integrated rate equation of Zero Order Reaction.
EASY
Physical Sciences>Matter and Its Interactions>Apply scientific principles and evidence to provide an explanation about the effects of changing the temperature or concentration of the reacting particles on the rate at which a reaction occurs.>Chemical Reactions - Chemical processes, their rates, and whether or not energy is stored or released can be understood in terms of the collisions of molecules and the rearrangements of atoms into new molecules, with consequent changes in the sum of all bond energies in the set of molecules that are matched by changes in kinetic energy.
MEDIUM
Physical Sciences>Matter and Its Interactions>Apply scientific principles and evidence to provide an explanation about the effects of changing the temperature or concentration of the reacting particles on the rate at which a reaction occurs.>Chemical Reactions - Chemical processes, their rates, and whether or not energy is stored or released can be understood in terms of the collisions of molecules and the rearrangements of atoms into new molecules, with consequent changes in the sum of all bond energies in the set of molecules that are matched by changes in kinetic energy.
EASY
Physical Sciences>Matter and Its Interactions>Apply scientific principles and evidence to provide an explanation about the effects of changing the temperature or concentration of the reacting particles on the rate at which a reaction occurs.>Chemical Reactions - Chemical processes, their rates, and whether or not energy is stored or released can be understood in terms of the collisions of molecules and the rearrangements of atoms into new molecules, with consequent changes in the sum of all bond energies in the set of molecules that are matched by changes in kinetic energy.