HARD
JEE Main/Advance
IMPORTANT
Earn 100
Graph shows a hypothetical speed distribution for a sample of gas particle:( for )
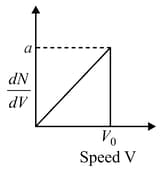

(a)The value of is
(b)The ratio is equal to
(c)The ratio is equal to
(d)Three fourth of the total particle has a speed between and
50% studentsanswered this correctly

Important Questions on Kinetic Theory of Gases
MEDIUM
JEE Main/Advance
IMPORTANT
HARD
JEE Main/Advance
IMPORTANT
MEDIUM
JEE Main/Advance
IMPORTANT
An oxygen cylinder of volume litres has an initial gauge pressure of and a temperature of . After some oxygen is withdrawn from the cylinder, the gauge pressure drops to and its temperature drops to . Estimate the mass of oxygen taken out of the cylinder ( , molecular mass of ).
EASY
JEE Main/Advance
IMPORTANT
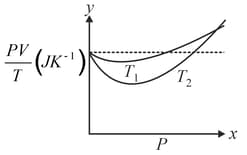
What is the value of where the curves meet on the -axis?
MEDIUM
JEE Main/Advance
IMPORTANT
(i) How many degrees of freedom do gas molecules have?
(ii) Obtain the work done by the gas expansion as a function of the initial pressure and volume . [Take
EASY
JEE Main/Advance
IMPORTANT
HARD
JEE Main/Advance
IMPORTANT
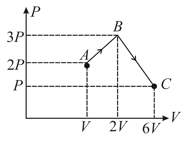
HARD
JEE Main/Advance
IMPORTANT
