EASY
11th CBSE
IMPORTANT
Earn 100
can be represented by structures and shown below. Can these two structures be taken as the canonical forms of the resonance hybrid representing ? If not, give reasons for the same.
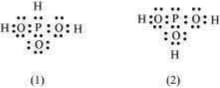

Important Questions on Chemical Bonding and Molecular Structure
MEDIUM
11th CBSE
IMPORTANT
Write the resonance structures for and .
MEDIUM
11th CBSE
IMPORTANT
Write the resonance structures for .
MEDIUM
11th CBSE
IMPORTANT
Write the resonance structures for .
EASY
11th CBSE
IMPORTANT
Use Lewis symbols to show electron transfer between the following atoms to form cations and anions :
and
EASY
11th CBSE
IMPORTANT
Use Lewis symbols to show electron transfer between the following atoms to form cations and anions :
and
EASY
11th CBSE
IMPORTANT
Use Lewis symbols to show electron transfer between the following atoms to form cation and anion:
and
MEDIUM
11th CBSE
IMPORTANT
Although both and are triatomic molecules, the shape of molecule is bent while that of is linear. Explain this on the basis of dipole moment.
EASY
11th CBSE
IMPORTANT
Write the significance/applications of dipole moment.
