MEDIUM
MHT-CET
IMPORTANT
Earn 100
Heat capacity of a substance is infinite. It means
(a)heat is given out.
(b)heat is taken in.
(c)no change in temperature whether heat is taken in or given out.
(d)change in temperature is infinite.
66.67% studentsanswered this correctly

Important Questions on Thermal Properties of Matter
EASY
MHT-CET
IMPORTANT
EASY
MHT-CET
IMPORTANT
EASY
MHT-CET
IMPORTANT
MEDIUM
MHT-CET
IMPORTANT
For an ideal monatomic gas, the universal gas constant $\mathrm{R}$ is $\mathrm{n}$ times the molar heat capacity at constant pressure $C_{p} .$ Here $n$ is
EASY
MHT-CET
IMPORTANT
When an ideal monatomic gas is heated at constant pressure, fraction of heat energy supplied which increases the internal energy of gas is,
MEDIUM
MHT-CET
IMPORTANT
Variation of molar specific heat of a metal with temperature (Temp.) is best depicted by the graph,
EASY
MHT-CET
IMPORTANT
Two circular discs $A$ and $B$ with equal radii are blackened. These are being cooled under identical circumstances. What inference can be drawn from their cooling curves?
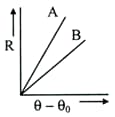
EASY
MHT-CET
IMPORTANT
The graph represents