Heat is supplied to a certain homogenous sample of matter, at a uniform rate. Its temperature is plotted against times as shown.
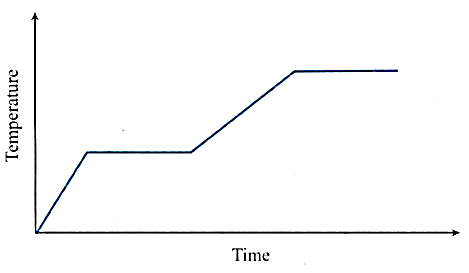
Which of the following conclusions can be drawn?
Important Questions on Thermometry, Thermal Expansion and Calorimetry
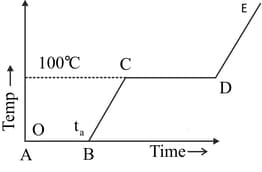
Refer to the plot of temperature versus time (Figure) showing the changes in the state of ice on heating (not to scale). Which of the following is correct?
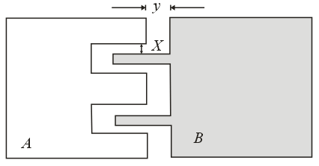
Two metal plates and made of same material are placed on a table as shown in the figure. If the plates are heated uniformly, the gaps indicated by and in the figure,
A bolt is passed through a pipe and a nut is just tightened. Coefficients of linear expansion for bolt and pipe material are and respectively. If the assembly is heated then:
of steam at melts to how many grams of ice at ? (Latent heat of ice and latent heat of steam )
of ice at is mixed with of water at in an insulating vessel having a negligible heat capacity. Calculate the final mass of water (in ) remaining in the container.
