MEDIUM
NEET
IMPORTANT
Earn 100
Heat treatment of muscular pain involves radiation of wavelength of about Which spectral line of H-atom is suitable for this purpose?
(a)Paschen ,
(b)Paschen,
(c)Balmer,
(d)Lyman,
100% studentsanswered this correctly

Important Questions on Structure of Atom
MEDIUM
NEET
IMPORTANT
MEDIUM
NEET
IMPORTANT
MEDIUM
NEET
IMPORTANT
MEDIUM
NEET
IMPORTANT
MEDIUM
NEET
IMPORTANT
HARD
NEET
IMPORTANT
MEDIUM
NEET
IMPORTANT
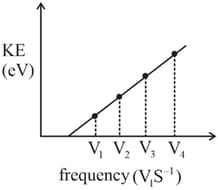
In a photoelectric experiment, the kinetic energy of photoelectrons was plotted against the frequency of incident radiation , as shown in the figure. Which of the following statements is correct?
MEDIUM
NEET
IMPORTANT
