Henry's law is valid for
A) Ammonia gas dissolution in water
B) gas dissolution in unsaturated blood
C) dissolution in water
D) dissolution in water
Important Questions on Solutions
The oxygen dissolved in water exerts a partial pressure of in the vapour above water. The molar solubility of oxygen in water is ______
(Round off to the Nearest Integer).
[Given : Henry's law constant for Density of water with dissolved oxygen]
gas is bubbled through water during a soft drink manufacturing process at . If exerts a partial pressure of then of would dissolve in of water. The value of is _______. (Nearest integer)
(Henry's law constant for at is )
Henry's constant (in kbar) for four gases and in water at is given below :
(density of water at ) This table implies that :

The gas with the highest value of Henry's law constant is
Aquatic animals are more comfortable in cold water than in warm water.
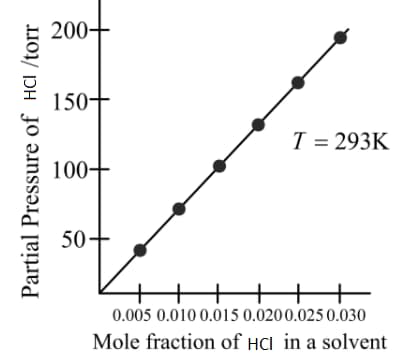
From the graph, the value of Henry's constant for the solubility of gas in cyclohexane is