MEDIUM
Earn 100
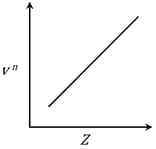
Henry Moseley studied characteristic -ray spectra of elements. The graph which represents his observation correctly is
Given Frequency of X-ray emitted
Atomic number
(a)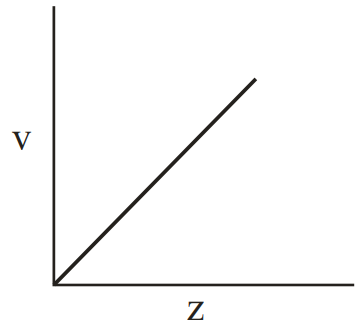

(b)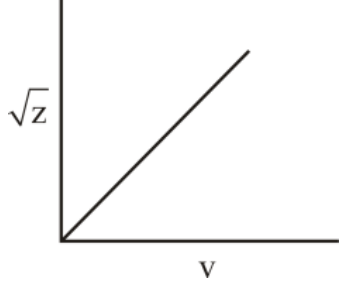

(c)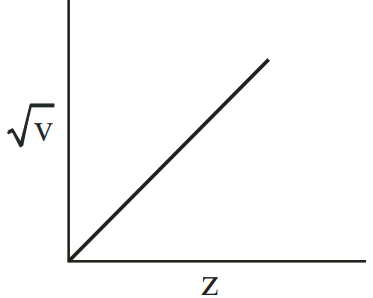

(d)

62.2% studentsanswered this correctly
Important Questions on Classification of Elements and Periodicity in Properties
EASY
The electronic configurations of Eu (Atomic no. ), Gd (Atomic no. ) and Tb (Atomic no. ) are:
MEDIUM
Which one of the following statements for D.I. Mendeleev, is incorrect ?
MEDIUM
In which of the following options, the law of triad is applicable?
EASY
Match List - I with List - II
| LIST-I (Atomic number) |
LIST-II (Block of periodic table) |
||
| (A) | I. | -block | |
| (B) | II. | -block | |
| (C) | III. | -block | |
| (D) | IV. | -block | |
Choose the correct answer from the options given below:
MEDIUM
It is observed that characteristic X-ray spectra of elements show regularity. When frequency to the power 'n' i.e. of X-rays emitted is plotted against atomic number , the following graph is obtained.
The value of 'n' is
MEDIUM
The electronic configurations of bivalent europium and trivalent cerium are:
(atomic number: , ,
MEDIUM
Which of the given atoms has the greatest electron affinity?
EASY
The last element of the - block in period is represented by the outermost electronic configuration:
EASY
Mendeleev's periodic law states that the properties of elements are a periodic function of their
EASY
The atomic number of the element unnilennium is :
MEDIUM
The pair of lanthanides with exceptionally high ionisation enthalpy than neighbouring elements:
MEDIUM
How do you appreciate the special nature of Inert gases?
EASY
Identify the incorrect match.
| Name IUPAC | Official Name |
| (a) Unnilunium | (i) Mendelevium |
| (b) Unniltrium | (ii) Lawrencium |
| (c) Unnilhexium | (iii) Seaborgium |
| (d) Unununium | (iv) Darmstadtium |
EASY
The outermost subshell electronic configuration of an element is .
Write the atomic number of the element.
MEDIUM
Give scientific reasons:
Elements belonging to the same group have the same valency.
EASY
Element with valence shell-electronic configuration as is placed in:
EASY
Which set does not show the correct match of ion and electronic configuration?
EASY
Match Column I (atomic number of elements) with Column II (position of elements in Periodic Table) and select the correct answer using the codes given below the Columns.
| Column I | Column II | ||
|---|---|---|---|
| A. | 19 | i. | p-block |
| B. | 22 | ii. | f-block |
| c. | 32 | iii. | d-block |
| d. | 64 | iv. | s-block |
EASY
The inert gas present in the second long period is