EASY
JEE Main
IMPORTANT
Earn 100
Higher order (>3) reactions are rare due to:
(a)Loss of active species on collision.
(b)Low probability of simultaneous collision of all the reacting species.
(c)Increase in entropy and activation energy as more molecules are involved.
(d)Shifting of equilibrium towards reactants due to elastic collisions.
60% studentsanswered this correctly

Important Questions on Chemical Kinetics
MEDIUM
JEE Main
IMPORTANT
The number of molecules with energy greater than the threshold energy for a reaction increases five fold by a rise of temperature from to . Its energy of activation in is _________ (Take )
MEDIUM
JEE Main
IMPORTANT
If of a first order reaction was completed in minutes, of the same reaction would be completed in approximately (in minutes) _____
(Take: )
MEDIUM
JEE Main
IMPORTANT
Decomposition of follows a first order reaction. In fifty minutes the concentration of decreases from to in one such decomposition. When the concentration of reaches , the rate of formation of will be:
MEDIUM
JEE Main
IMPORTANT
For the reaction the rate constant (in ) is given by
The energy of activation in is ________ . (Nearest integer)
[Given : ]
MEDIUM
JEE Main
IMPORTANT
According to the following figure, the magnitude of the enthalpy change of the reaction
in
is equal to __________ . (Integer answer)
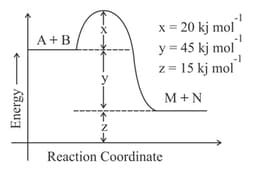
MEDIUM
JEE Main
IMPORTANT
For a first order reaction, the ratio of the time for completion of a reaction to the time for completion is ___________ . (Integer answer)
EASY
JEE Main
IMPORTANT
For the reaction , which statement is correct?
EASY
JEE Main
IMPORTANT
It is true that:
