MEDIUM
Earn 100
How do metals react with water?
Important Questions on Sorting Materials into Groups
EASY
EASY
MEDIUM
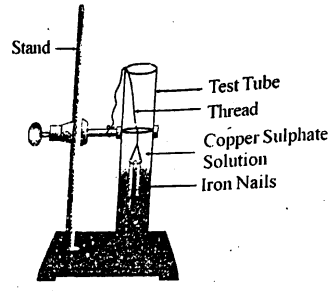
Write a chemical equation for the reaction taking place in the test tube. How does the colour of the solution change? What is the change in colour of iron nails?
EASY
EASY
MEDIUM
EASY
HARD
EASY
HARD
MEDIUM
MEDIUM
MEDIUM
MEDIUM
EASY
What is your observation in the following case:
Burning of magnesium in air.
EASY
MEDIUM
EASY
MEDIUM
MEDIUM

