EASY
Earn 100
How do you explain with the help of a graph, the increase in the value of molar conductivity with dilution in case of strong and weak electrolyte?
Important Questions on Electrochemistry
EASY
EASY
: Conductivity always increases with decreases in the concentration of electrolyte.
: Molar conductivity always increases with decreases in the concentration of electrolyte.
The correct option among the following
EASY
HARD
MEDIUM
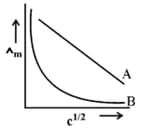
In the plot of molar conductivity vs square root of concentration , following curves are obtained for two electrolytes A and B :
Answer the following :
What happens on extrapolation of to concentration approaching zero for electrolytes A and B ?
MEDIUM
Given below are two statements:
Statement I: For , molar conductivity increases steeply with dilution.
Statement II: For carbonic acid, molar conductivity increases slowly with dilution. In the light of the above statements, choose the correct answer from the options given below
MEDIUM
EASY
EASY
EASY
MEDIUM
MEDIUM
EASY
[Given: equivalent conductance at infinite dilution of and ]
MEDIUM
EASY
MEDIUM
Conductivity of dilute hydrochloric acid is greater than that of acetic acid.
MEDIUM
EASY
MEDIUM
MEDIUM