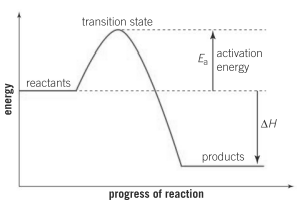
How does the total amount of energy contained in the reactants compare to the products in Haber's process for the manufacture of ammonia? Which substances will be more energetically stable, reactants or products?


Important Questions on Balance
Industrial reaction conditions for the production of ammonia is as shown below in the graph:
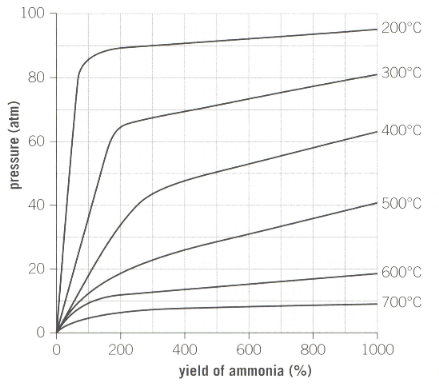
What temperature achieves the highest yield of ammonia?
Industrial reaction conditions for the production of ammonia is as shown below in the graph:
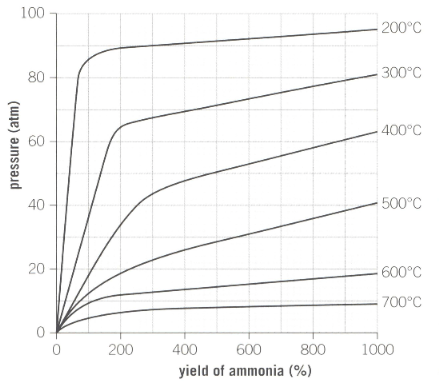
Describe how the yield changes as the pressure is increased.
Industrial reaction conditions for the production of ammonia is as shown below in the graph:
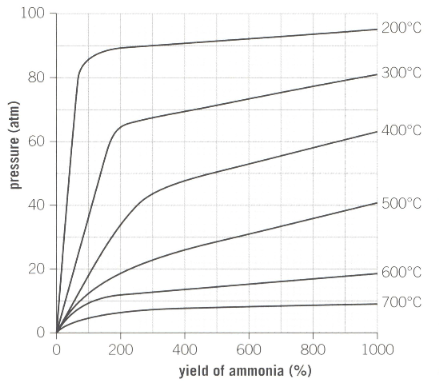
The ideal temperature used by Haber is approximately . Outline the reasons why a higher temperature would be used to increase production of ammonia instead of a lower temperature.
Industrial reaction conditions for the production of ammonia is as shown below in the graph:
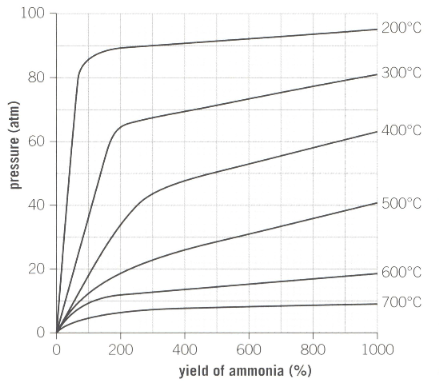
A pressure of is used during the process. Why does industry not use a much higher pressure to maximise yield?
Industrial reaction conditions for the production of ammonia is as shown below in the graph:
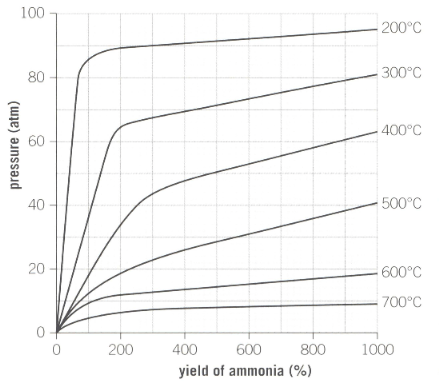
Explain with reference to the position of the equilibrium why increasing the pressure of this closed system favours the forward reaction.
