EASY
10th Telangana Board
IMPORTANT
Earn 100
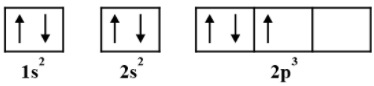
How many maximum numbers of electrons can be accommodated in an orbital?
100% studentsanswered this correctly

Important Questions on Structure of Atom
MEDIUM
10th Telangana Board
IMPORTANT
MEDIUM
10th Telangana Board
IMPORTANT
MEDIUM
10th Telangana Board
IMPORTANT
Which is the outermost shell?
MEDIUM
10th Telangana Board
IMPORTANT
How many electrons are there in its outermost shell?
MEDIUM
10th Telangana Board
IMPORTANT
What is the atomic number of the element?
HARD
10th Telangana Board
IMPORTANT
Write the electronic configuration of the element.
MEDIUM
10th Telangana Board
IMPORTANT
EASY
10th Telangana Board
IMPORTANT