MEDIUM
JEE Main/Advance
IMPORTANT
Earn 100
How many of the following atoms have maximum ionisation energy than boron?
(i) (ii) (iii) (iv) (v) (vi)
44.86% studentsanswered this correctly

Important Questions on Classification of Elements and Periodicity in Properties
MEDIUM
JEE Main/Advance
IMPORTANT
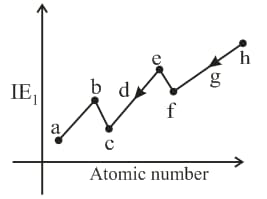
Where are period elements. If the difference between the atomic numbers of elements and is and the difference between the atomic number of elements and is What is the value of
EASY
JEE Main/Advance
IMPORTANT
Values of of an element are and Predict group number in Modern Periodic Table.
EASY
JEE Main/Advance
IMPORTANT
Electron gain enthalpy of is What is the value of
MEDIUM
JEE Main/Advance
IMPORTANT
The electron gain enthalpy of a hypothetical element is per atom. How much energy in is released when of are completely converted to , ions in gaseous state ? (Molar mass of )
EASY
JEE Main/Advance
IMPORTANT
What is atomic number of element which have maximum electron affinity in Modern Periodic table.
MEDIUM
JEE Main/Advance
IMPORTANT
How many of the following elements are more electronegative than Boron.
(i) (ii) (iii) (iv) (v) (vi) (vii)
MEDIUM
JEE Main/Advance
IMPORTANT
The group in modern periodic table in which all the elements do not have the same number of electrons in their outermost shell is: (considering upto period)
EASY
JEE Main/Advance
IMPORTANT
Element corresponding to which of these/this atomic number belongs to -block in Modern Periodic Table:
