MEDIUM
JEE Main/Advance
IMPORTANT
Earn 100
How many of the following reactions yield ?
(i) (ii) (iii)
(iv) (v) (vi)
(vii) (viii) (ix)
50% studentsanswered this correctly

Important Questions on The p-Block Elements
MEDIUM
JEE Main/Advance
IMPORTANT
| Column I | Column II | ||
| (a) | (p) | One of the products is a mixed anhydride. | |
| (b) | (q) | One of the products is an acidic oxide. | |
| (c) | (r) | The oxidation state of the central atom of one of the products is . | |
| (d) | (s) | One of the products is a colourless paramagnetic gas. |
MEDIUM
JEE Main/Advance
IMPORTANT
EASY
JEE Main/Advance
IMPORTANT
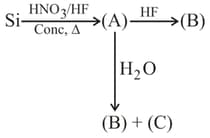
Select the correct option:
MEDIUM
JEE Main/Advance
IMPORTANT
gives following colour with flame.
MEDIUM
JEE Main/Advance
IMPORTANT
Which of the following around is correct.
HARD
JEE Main/Advance
IMPORTANT
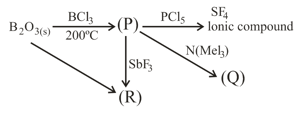
Select the correct option?
MEDIUM
JEE Main/Advance
IMPORTANT
MEDIUM
JEE Main/Advance
IMPORTANT
