EASY
Earn 100
How many statements are true for the following pair of compounds?
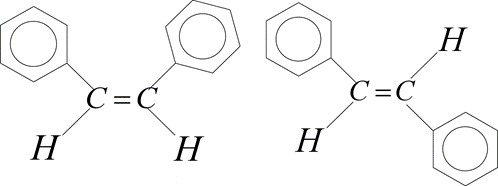
Cis Trans
(i) The dipole moment of trans isomer is zero
(ii) The boiling point of cis isomer is more than trans isomer
(iii) Cis isomer is more stable than the trans isomer
(iv) These are also called configurational diastereomers
(v) These are readily interconvertible under normal conditions
(vi) The melting point of trans isomer is more than the cis isomer
(vii) Trans isomer is more soluble than cis isomer in polar solvents
50% studentsanswered this correctly
Important Questions on Chemical Bonding and Molecular Structure
EASY
MEDIUM
EASY
EASY
HARD
MEDIUM
HARD
HARD
EASY
EASY
EASY
EASY
EASY
EASY
EASY
HARD
EASY
MEDIUM
HARD
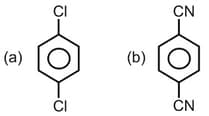
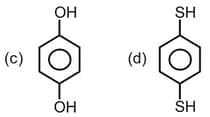
EASY

