MEDIUM
Upper Secondary: IGCSE
IMPORTANT
Earn 100
How many times greater is the mass of a proton than the mass of an electron?

Important Questions on The Nuclear Atom
MEDIUM
Upper Secondary: IGCSE
IMPORTANT
What is the same for the atoms of two different isotopes of an element? What is different for them?
MEDIUM
Upper Secondary: IGCSE
IMPORTANT
Copy one of the diagrams of an atom shown in the figure. Add the following labels to your drawing: Electron, neutron, proton, nucleus.
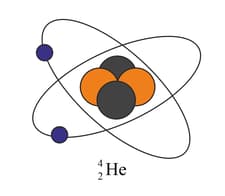
HARD
Upper Secondary: IGCSE
IMPORTANT
A specific nucleus may be represented like this: . Copy and complete the table to explain what the three symbols mean.
| Symbol | Name | What it tells us |
| X | ||
| Z | ||
| A |
MEDIUM
Upper Secondary: IGCSE
IMPORTANT
Write a word equation linking the numbers of protons, neutrons and nucleons in a nucleus.
MEDIUM
Upper Secondary: IGCSE
IMPORTANT
Atoms of an element can be different isotopes of that element. Copy and complete the sentences below, writing either the words the same number or the words different numbers in the gaps.
Two isotopes of an element have_____ of neutrons in their nuclei.
MEDIUM
Upper Secondary: IGCSE
IMPORTANT
Atoms of an element can be different isotopes of that element. Copy and complete the sentences below, writing either the words the same number or the words different numbers in the gaps.
Two isotopes of an element have _____ of protons in their nuclei.
MEDIUM
Upper Secondary: IGCSE
IMPORTANT
Atoms of an element can be different isotopes of that element. Copy and complete the sentences below, writing either the words the same number or the words different numbers in the gaps.
Two isotopes of an element have _____ of nucleons in their nuclei.
MEDIUM
Upper Secondary: IGCSE
IMPORTANT
Diamond is a form of carbon. It is made up (almost entirely) of carbon - Atom. The symbol for the nucleus of a carbon - atom is .
How many protons are there in a carbon - atom?
