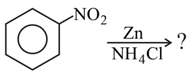
MEDIUM
Earn 100
How nitrobenzene reacts with lithium aluminium hydride?
Important Questions on Organic Compounds Containing Nitrogen
EASY
In the reaction given below, is
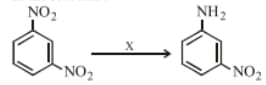
HARD
Write structural formula of the compounds to .
MEDIUM
HARD
(a) Nitrobenzene to nitrobenzoic acid
HARD
Supply the structures to the bracketed compounds to
(c) 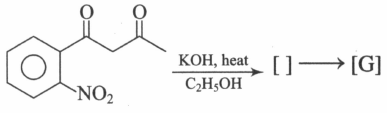
HARD
MEDIUM
HARD
Give balanced chemical equation of reduction of nitrobenzene in neutral medium.
HARD
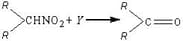
MEDIUM
HARD
Hydrazobenzene is formed when nitrobenzene is reduced with:
MEDIUM
Azoxybenzene can be obtained by the treatment of nitrobenzene with
HARD
Supply the structures to the bracketed compounds to
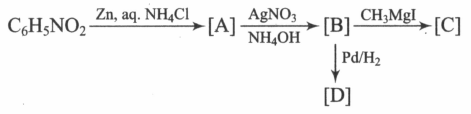
MEDIUM
HARD
MEDIUM
MEDIUM
MEDIUM
Predict the major product of the following reaction.
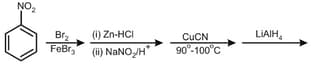
EASY