EASY
Earn 100
How to choose an indicator for an acid base titration?
Important Questions on Consequences
HARD
EASY
MEDIUM
EASY
Write the quadrants in which the following points lie:
MEDIUM
MEDIUM
How many of the following can turn moist red litmus blue?
$\mathrm{H}_{3} \mathrm{BO}_{3}, \mathrm{Na}_{2} \mathrm{B}_{4} \mathrm{O}_{7} \cdot 10 \mathrm{H}_{2} \mathrm{O}, \mathrm{CaCO}_{3}, \mathrm{Na}_{2} \mathrm{CO}_{3},$ Potash alum, $\mathrm{SnO}_{2}, \mathrm{NaNO}_{3}, \mathrm{Na}_{3} \mathrm{PO}_{4}, \mathrm{NaAlO}_{2}$
EASY
An element reacts with water to form a solution which turns phenolphthalein solution pink. The element is most likely to be:
MEDIUM
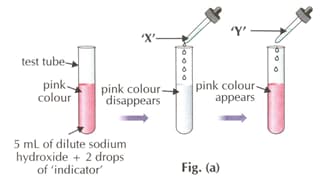
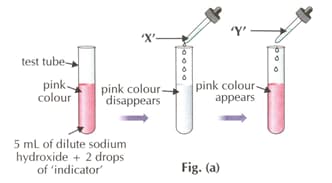
Which of the following indicators is used in the activity?
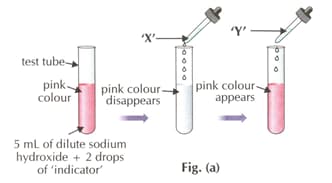
HARD
MEDIUM
HARD
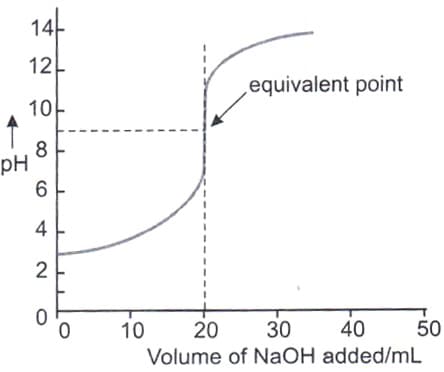
KIn value of phenolphthalein is 4.0 x 10-10. Thus, incorrect statement is
MEDIUM
HARD
MEDIUM
EASY
HARD
HARD
HARD
Phenolphthalein