HARD
Earn 100
How to find out the charges of polyatomic ions?
Important Questions on Chemical Bonding and Molecular Structure
EASY
MEDIUM
MEDIUM
Give a reason for the following:
Ionic compounds have a high melting point.
MEDIUM
MEDIUM
EASY
EASY
EASY
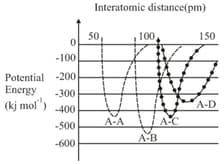
EASY
MEDIUM
EASY
EASY
EASY
MEDIUM
MEDIUM
MEDIUM
Two elements and have electronegativities and respectively. The nature of bond between and would be
MEDIUM
HARD
MEDIUM
MEDIUM
Match the following species with the correct number of electrons present in them:
| Species | Number of Electrons |

