How to represent probability of finding an electron?
Important Questions on Quantum and Nuclear Physics (HL)
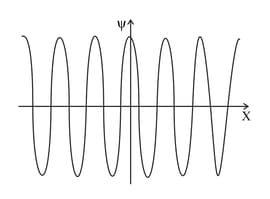
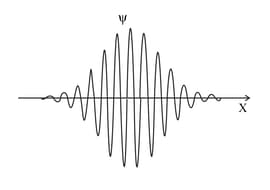
The graphs represent the wave functions of two electrons. Identify the electron with the least uncertainty in the position.
Represent the union of two sets by Venn diagram for each of the following.
is a prime number between and
is an odd number between and
According to the Copenhagen interpretation, system's wave function is a superposition of all possible states.
The graphs represent the wave functions of two electrons. Identify the electron with the least uncertainty in momentum.
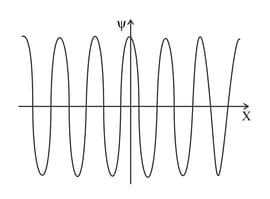
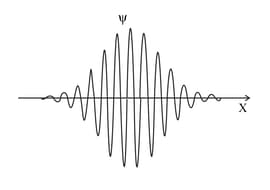
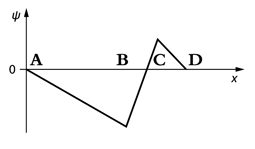
The graph shows the wavefunction of a particle. Near which position is the particle most likely to be found?
Theoretically it is possible in principle to balance a pencil on its tip so that it stands vertically on a horizontal table. Explain why in quantum theory this is impossible in principle. (You can turn this problem into a good theoretical extended if essay you try to estimate the time the pencil will stay up after it has been momentarily balanced!)

