How will you distinguish between the following pairs of terms?
Hexagonal close packing and cubic close packing.
Important Questions on The Solid State
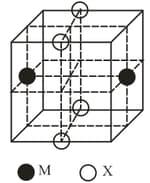
The cubic unit cell structure of a compound containing cation and anion is shown below. When compared to the anion, the cation has smaller ionic radius. Choose the correct statement(s).

(a) Aluminium crystallizes in a cubic close-packed structure. Its metallic radius is . What is the length of the side of the unit cell?
(b) Why is potassium chloride sometimes violet instead of pure white?
What is the number of atoms in end centered unit cell?
A two dimensional solid is made by alternating circles with radius and such that the sides of the circles touch. The packing fraction is defined as the ratio of the area under the circles to the area under the rectangle with sides of length and
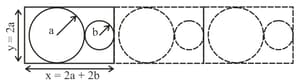
The ratio for which the packing fraction is minimized is closest to:

