How would you change a metal like into its ions?

Important Questions on Electrolysis
How would you change ion to ?
A solution of caustic soda in water or when fused, conducts an electric current. What is the similarity in these two cases?
During electrolysis of an aqueous solution of sulphuric acid between platinum electrodes, two types of anions migrate towards the anode but only one of them is discharged. Name the two anions.
During electrolysis of an aqueous solution of sulphuric acid between platinum electrodes, two types of anions migrate towards the anode but only one of them is discharged:
Name the main product of the discharge of anion at the anode and write the anode reaction.
During electrolysis of an aqueous solution of sulphuric acid between platinum electrodes, name the product at the cathode and write the reaction.
During electrolysis of an aqueous solution of sulphuric acid, do you notice any change in colour. State why?
Why electrolysis of an aqueous solution of sulphuric acid, is considered as an example of catalysis?
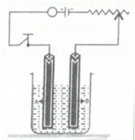
Copper sulphate solution is electrolysed using a platinum anode.
Study the diagram given alongside and answer the following questions:
Give the names of the electrodes A and B.