HARD
Earn 100
Hybridization involved in is
(a)
(b)
(c)
(d)
50% studentsanswered this correctly
Important Questions on The p-Block Elements
EASY
EASY
MEDIUM
EASY
MEDIUM
EASY
| Column I | Column II |
| a. | (i) T-shape |
| b. | (ii) Pentagonal bipyramidal |
| c. | (iii) Linear |
| d. | (iv) Square – pyramidal |
| (v) Tetrahedral |
MEDIUM
MEDIUM
EASY
EASY
MEDIUM
MEDIUM
EASY
EASY
HARD
EASY
EASY
EASY
(i)
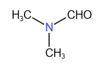 (ii)
(ii) 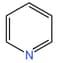
(iii) (iv)
MEDIUM
EASY

