Hydrazine used in rocket fuels can be obtained by the reaction of ammonia and hydrogen peroxide according to the following equations
If (formation) of and are and respectively, for the decomposition of hydrazine into and is

Important Questions on Thermodynamics
One mole of Ideal gas . follow the process as shown in figure. Predict the following:
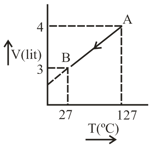
Calculate work done, Heat of process.
(a) What is the final temperature?
(b) What is work done? Given and for
A certain mass of a gas initially at is expanded reversibly and isothermally to a final volume of
(a) calculate work done by the gas and heat supplied in this process to the gas.
(b) Now, if the gas is restored to initial position by compressing it using an external constant pressure of atm. Find work done on the gas in this process and heat rejected by gas
(c) the above two processes, what is the net heat gained by surroundings?
[Note: From above question see that surroundings has done extra work on the system but system has returned that work in the form of heat to surroundings and work is considered on organised form of energy while heat as an unorganised form hence in the above process, there must be net increment in randomness of universe which will be called Entropy, soon.]
One mole of monoatomic gas was taken through a cyclic process as shown in the figure. Calculate
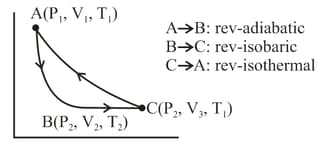
when the process is carried out reversibly?
when the process carried out irreversibly (one step)
An exothermic reaction is always thermodynamically spontaneous.
