HARD
Earn 100
Hydrazine was taken in a constant volume container at 27oc and 1.0 atm and equal moles of oxygen gas were introduced, sealed and heated following equlibria established
The gaseous mixture on passing through moisture absorbent, a decrease of 360 mm in equilibrium pressure observed. The dried gaseous mixture passed through Ammonia absorber, a further decrease of 20 mm in equilibrium pressure observed. After calculating Kp2, report the value of Kp3
(a)2.89 × 10–3
(b)3.24 × 10–2
(c)4.13 × 103
(d)5.96 × 102
50% studentsanswered this correctly
Important Questions on Chemical Equilibrium
HARD
MEDIUM
MEDIUM
MEDIUM
EASY
MEDIUM
MEDIUM
MEDIUM
The stoichiometry and solubility product of a salt with the solubility curve given below is, respectively:
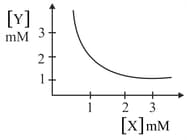
EASY
HARD
MEDIUM
HARD
MEDIUM
HARD
MEDIUM
Which of the following choices is correct for a mixture of and
EASY
MEDIUM
HARD
HARD
MEDIUM
[Solubility product for ]

