Hydrochloric acid is used to purify bone black.
Important Questions on Group 17 Elements : Halogen Family
Write the equations for reactions of chlorine with the following.
Excess
In which form of complex, platinum is dissolved in aqua regia?
What is Aqua regia? Give its reaction with Gold.
Hydrogen chloride gas is prepared in the laboratory using concentrated sulphuric acid and sodium chloride. Answer the question that follows based on this reaction:
- Name the drying agent not used for drying the gas.
Assertion (A): gas is dried by passing through concentrated .
Reason (R): gas reacts with that gives white fumes
Write the balanced chemical equation for the laboratory preparation of hydrogen chloride gas.
Hydrogen chloride gas is prepared in the laboratory using concentrated sulphuric acid and sodium chloride. Answer the question that follows based on this reaction:
- How is the gas collected?
Hydrogen chloride gas is prepared in the laboratory using concentrated sulphuric acid and sodium chloride. Answer the question that follows based on this reaction:
- Give the balanced chemical equation for the reaction with suitable condition(s) if any.
Hydrogen chloride gas is prepared in the laboratory using concentrated sulphuric acid and sodium chloride. Answer the question that follows based on this reaction:
- Why is concentrated sulphuric acid used instead of concentrated nitric acid?
State one relevant reason for the following:
- Hydrogen chloride gas fumes in moist air.
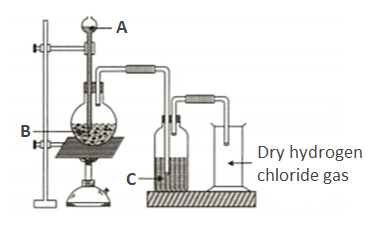
Write down the balanced chemical equation for the reaction between and that takes place upon heating.
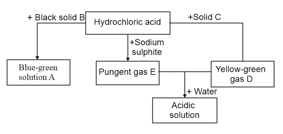
Write the names of the substances to .
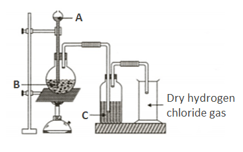
Name the liquid and its function.

