Hydrogen atom in its ground state is excited by means of monochromatic radiation of wave length . How many different lines are possible in the resulting spectrum? Calculate the longest wavelength among them. You may assume the ionization energy of hydrogen atom as .

Important Questions on Atomic Physics
An electron in the ground state of hydrogen atoms is revolving in anti clock wise direction in a circular orbit of radius .
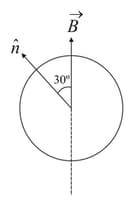
(i) Obtain an expression for the orbital magnetic dipole moment of the electron.
(ii) The atom is placed in a uniform magnetic induction, such that the plane normal to the electron orbit makes an angle of with the magnetic induction. Find the torque experienced by the orbiting electron.
A neutron of kinetic energy collides inelastically with a singly ionized helium atom at rest. It is scattered at an angle of with respect of its original direction.
(a) Find the allowed values of the energy of the neutron and that of the atom after the collision.
(b) If the atom gets de-excited subsequently by emitting radiation, find the frequencies of the emitted radiation. [Given: Mass of atom = (mass of neutrons Ionization energy of atom ]
A positronium consists of an electron and a positron revolving about their common centre of mass. Derive and calculate
(i) Separation between the electron and positron in their first excited state.
(ii) Kinetic energy of the electron in ground state.
