Hydrogen gas is not widely used as a reducing agent because
hydrogen decomposes to atomic hydrogen at higher temperature
hydrogen isomerises to ortho hydrogen at higher temperature
many metals form hydrides at lower temperature

Important Questions on Metals And Non-metals
Metals are refined by using different methods. Which of the following metals are refined by electrolytic refining?
(i)
(ii)
(iii)
(iv)
Four metals P, Q, R and S are tested with water, steam and dilute hydrochloric acid. The table given below shows the results of the experiment.
| Metals | Reaction with water | Reaction with steam | Reaction with dil. |
| P |  |
 |
 |
| Q |  |
 |
 |
| R |  |
 |
 |
| S |  |
 |
 |
Between which two metals should hydrogen be placed in the activity series?
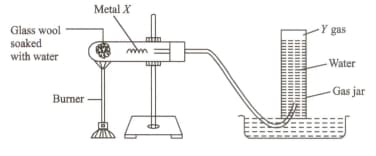
An experimental set up is shown in the figure. Identify the metal X and gas Y respectively in the figure.
An element X (atomic number ) reacts with another element Y (atomic number ) to form a compound Z. Which of the following statements are true regarding this compound?
I. Molecular formula of Z is
Il. It is soluble in water.
Ill. X and Y are joined by sharing of electrons.
IV. It would conduct electricity in the molten state.
There are four metals K, L, M and N. Identify them by using the hints given below.
K forms basic oxide.
L forms amphoteric oxide.
Oxide of M dissolves in water to form alkali.
N does not react with water at all.
Which one of the following metal oxides shows both acidic and basic characters?
