Hydrogen gas is not widely used as a reducing agent because _____.
temperatures.

Important Questions on Chemistry
Consider the following statements related to diamond and graphite.
Both diamond and graphite are used as abrasives.
Diamond and graphite have different arrangements of carbon atoms.
The carbon atoms in graphite have a different number of neutrons from those in a diamond.
The carbon atoms in both graphite and diamond have four single covalent bonds.
The incorrect statement(s) is/are _____.
C4+ does not exist but Pb4+ exists although both belong to the same group. This is because _____ .
I. Size of carbon is much smaller than Pb.
II. Large amount of energy is needed in case of carbon.
III. Of inert pair effect.
IV nucleus cannot hold such a large number of electrons.
The correct statement (s) is/are:
The structures of three hydrocarbons are given below.
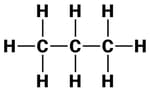
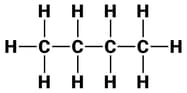
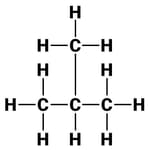
Which statement is correct for all the above three compounds?
The diagram shows the molecule, ethyl propanoate.
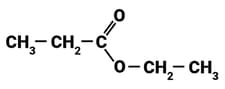
How many bonding pairs of electrons are there in the molecule?
