Ice of mass and at a temperature of is placed in a copper vessel heated to . The resultant mixture is of ice and water. Find the mass of the vessel. The specific heat capacity of copper .

Important Questions on Thermometry, Thermal Expansion and Calorimetry
When a small ice crystal is placed in overcooled water it begins to freeze instantaneously.
What amount of ice is formed from of water overcooled to of water and of water
When a small ice crystal is placed in overcooled water it begins to freeze instantaneously.
What should be the temperature of the overcooled water in order that all of it be converted into ice at
of water and of water
An electric heater whose power is is immersed in water in a calorimeter. In the water is heated by . What part of the energy of the heater passes out of the calorimeter in the form of radiant energy?
An ice cube whose mass is is taken from a refrigerator where its temperature was . If no heat is gained or lost from outside, how much water will freeze onto the cube if it is dropped into a beaker containing water at Latent heat of fusion , specific heat capacity of ice
Victoria Falls in Africa is in height. Calculate the rise in temperature of the water if all the potential energy lost in the fall is converted into heat.
Equal masses of three liquids and are taken. Their initial temperatures are and , respectively. When and are mixed the temperature of the mixture is . When and are mixed, the temperature of the mixture is . Find the temperature if all three are mixed.
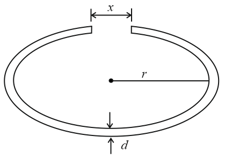
A cylindrical metal rod of length is shaped into a ring with a small gap as shown. On heating the system