EASY
Earn 100
Identify a strong electrolyte in the following compounds.
50% studentsanswered this correctly
Important Questions on Electrochemistry
EASY
Formic acid,
Acetic acid,
Benzoic acid.
EASY
MEDIUM
Conductivity of dilute hydrochloric acid is greater than that of acetic acid.
EASY
HARD
EASY
: Conductivity always increases with decreases in the concentration of electrolyte.
: Molar conductivity always increases with decreases in the concentration of electrolyte.
The correct option among the following
MEDIUM
EASY
The variation of molar conductively with concentration of an electrolyte (X) in aqueous solution is shown in the given figure.
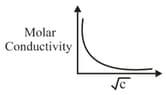
The electrolyte X is :
MEDIUM
EASY
[Given: equivalent conductance at infinite dilution of and ]
HARD
MEDIUM
MEDIUM
HARD
MEDIUM
MEDIUM
EASY
EASY
EASY
EASY
The group of compounds which dissociate partially in aqueous solutions is
